Morphological Phases in Ionic Liquids Induce High Conductivities at Low Temperatures
January 14, 2016
Scientific Achievement
By adjusting stiffness and steric interactions of the amphiphilic ionic liquid (IL) molecules, we showed that ion-conducting lamellar or 3D continuous phases can be obtained in dry IL melts at ambient temperatures.
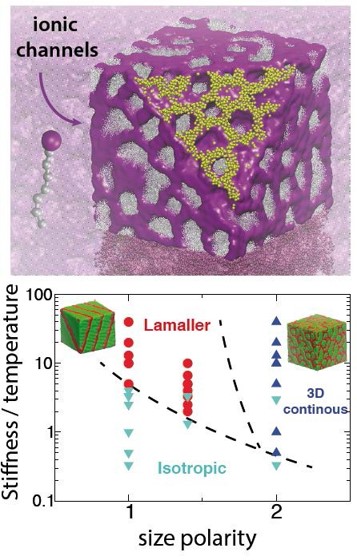 3D morphological phases with ion conducting channels (yellow spheres) emerge in ionic liquids with increasing size polarity along molecules. The stiffer the molecular tails are, the higher the ionic conductivity is.
3D morphological phases with ion conducting channels (yellow spheres) emerge in ionic liquids with increasing size polarity along molecules. The stiffer the molecular tails are, the higher the ionic conductivity is.
Significance and Impact
ILs have been emerging components in many electrochemical applications, particularly for energy storage (e.g., electrolytes in batteries). Our findings can help elucidate design principles for more efficient IL systems.
Research Details
- Used extensive molecular dynamics simulations in large scale model systems by considering both short and long-range Coulomb interactions.
- Investigated temperature dependence, properties of polymeric tail and excluded volume symmetry of the molecules, and formation of ionic channels in ILs without solvent.
Morphology-Enhanced Conductivity in Dry Ionic Liquids
Erbas, A., Olvera de la Cruz, M.
Phys. Chem. Chem. Phys. 18, 2016, 6441-6450.
Work performed at Northwestern University
