Superionics in Colloidal Crystals
November 3, 2022
Scientific Achievement
Crystal of asymmetric in size oppositely charged nanoparticles (NP) in equilibrium with a reservoir undergo ionic to metallic bonding transition where the small NP lattice melts and the crystals expand.
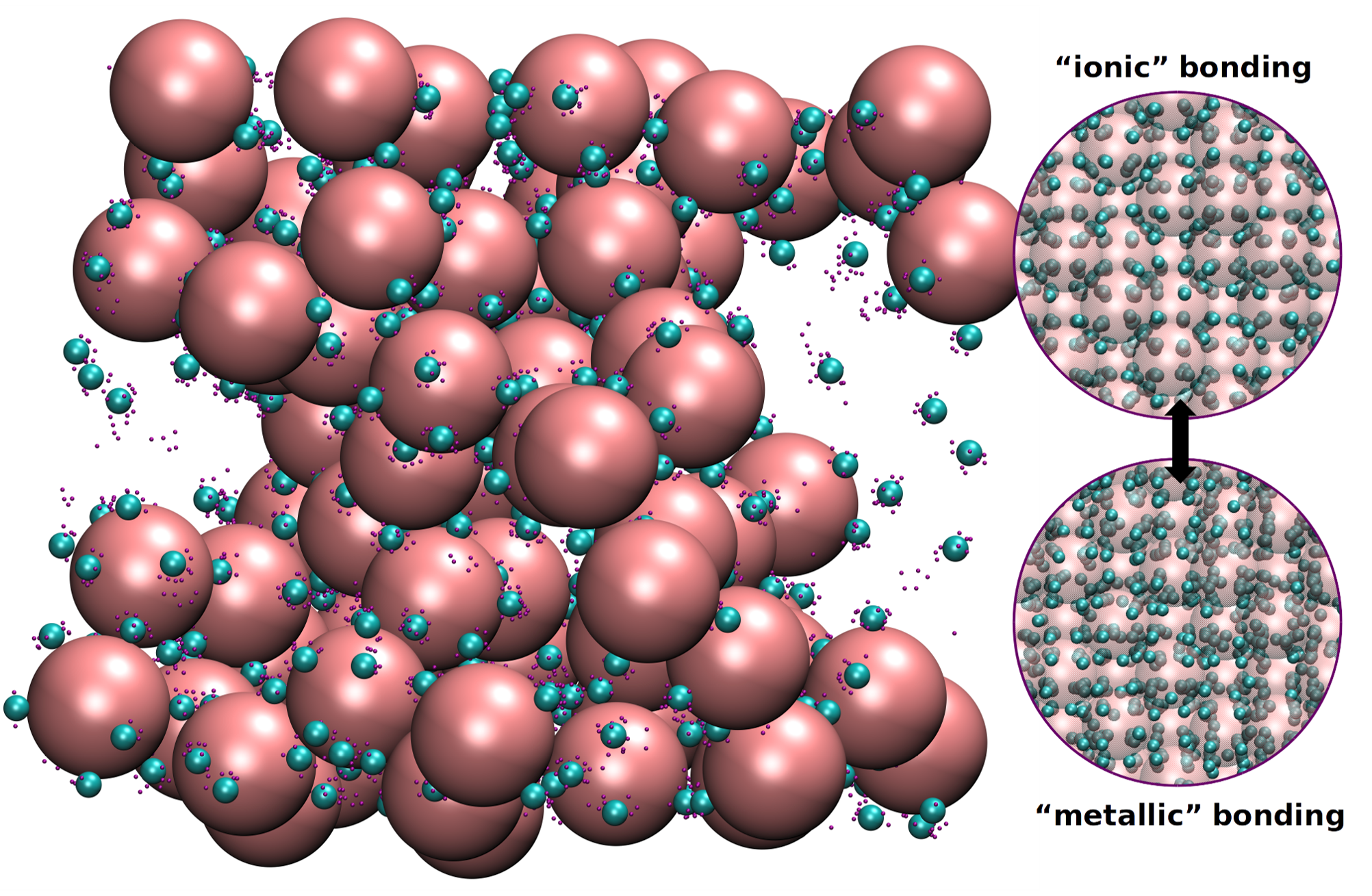
Increase of temperature and nanoparticle concentration in the reservoir change the bonding in the assemblies from ionic-like to metallic-like.
Significance and Impact
Provides insights on how to control the superionic transition and the crystal structure of charged nanoparticles via temperature and solution composition.
Research Details
- Molecular dynamics simulations are used to study the equilibrium between a binary-charged colloidal crystal with a particle reservoir using explicit ions and Coulomb interactions.
- Theory based on free energy calculations predicts a phase diagram of colloidal crystals in good match with simulations.
- Theoretical analysis reveals the driving force for the ionic-to-metallic bonding transition to be enthalpic.
Superionic Colloidal Crystals: Ionic to Metallic Bonding Transitions
Lin, Y.; Olvera de la Cruz, M.
Journal of Physical Chemistry B, 126(35), 2022, 6740-6749.
Work performed at Northwestern University
