Transitional Bonding in Colloidal Systems
Scientific Achievement
The discovery of a new type of bonding in colloidal systems where nanoparticles diffuse and hold together colloidal crystals in the same way electrons do in metallic bonding.
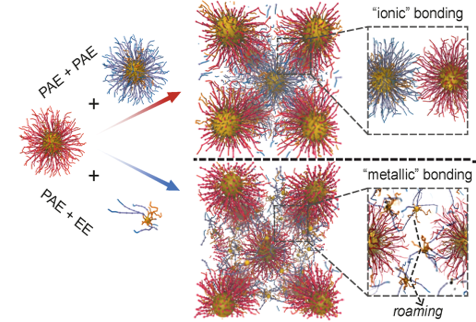
Snapshots from the MD simulation depicting “ionic” bonding behavior shown by PAE + PAE assemblies, and “metallic” bonding behavior shown by PAE + EE assemblies, where roaming EEs hold the crystal of repulsive PAEs together.
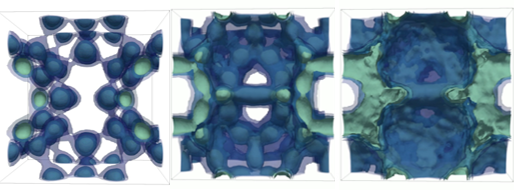
EEs cumulative probability of occupancy per unit cell (termed Boltzmann volumes) for EEs with 8 linkers in a Frank Kasper lattice when the ratio of EE:PAE is 4:1 (left) and when the ratio is 12:1 for 8 linkers (middle) and for 4 linkers (right). The larger the Boltzmann volume is the larger is the degree of delocalization of the EEs
Significance and Impact
Numerous colloidal compounds have been devised following design rules akin to ionic crystals, where individual DNA functionalized nanoparticles behave as programmable atom equivalents (PAEs) in superlattices. This electron-atom-equivalent duality lays the foundation to explore colloidal metallic alloy analogues.
Research Details
- We show PAEs that behave as electron-equivalents (EEs) when their sizes and DNA grafting density are reduced.
- In mixtures with large PAEs, the EEs roam the crystal as electrons in metals holding the large PAEs in specific lattices sites, akin to electron clouds in atomic metals.
- As the number of DNA strands increases or the temperature decreases, the EEs localize yielding a transition from metal to compound.
Particle Analogs of Electrons in Colloidal Crystals
Girard, M; Wang, S; Du, JS; Das, A; Huang, Z; Dravid, VP; Lee, B; Mirkin, CA; Olvera de la Cruz, M.
Science, 364, 2019, 1174-1178.
Work Performed at Northwestern University
